news
Introducing our reagents for your SARS-CoV-2 research
The SARS-CoV-2 coronavirus encodes a papain-like cysteine protease PLpro (NSP3) that cleaves the viral polyprotein, removes the ubiquitin-like protein ISG15 and (with lower activity) Lys48-linked polyubiquitin chains. As PLpro is essential for SARS-CoV-2 replication, it represents a promising target for the development of antiviral drugs. In light of this and to complement our well-known ubiquitin based reagents, we are expanding our portfolio of ISG15 based reagents.
Activity assay reagents. Fluorescence polarization assay reagent UbiQ-287 (short name hISG15 FP, Figure 1) is based on full-length human ISG15 that is linked via an isopeptide bond to a 5-carboxytetramethylrhodamine (TAMRA, exc/emi 550/590 nm) functionalized dipeptide. The fluorescence polarization assay reagent UbiQ-073 (short name ISG15 FP) is based on full-length mouse ISG15. In addition, we have UbiQ-127 (Ac-ISG15prox-Rh110MP, Figure 1) as a quenched, fluorescent substrate based on the C-terminal domain of mouse ISG15. Cleavage of the amide bond between the C-terminal Gly and rhodamine110 (Rh110) moiety releases the highly fluorescent Rh110-morpholinecarbonyl (Rh110MP). Overall, UbiQ-127 offers the excellent properties of a quenched Ubl-Rh110X substrate with a very high fluorescence intensity after proteolytic cleavage.
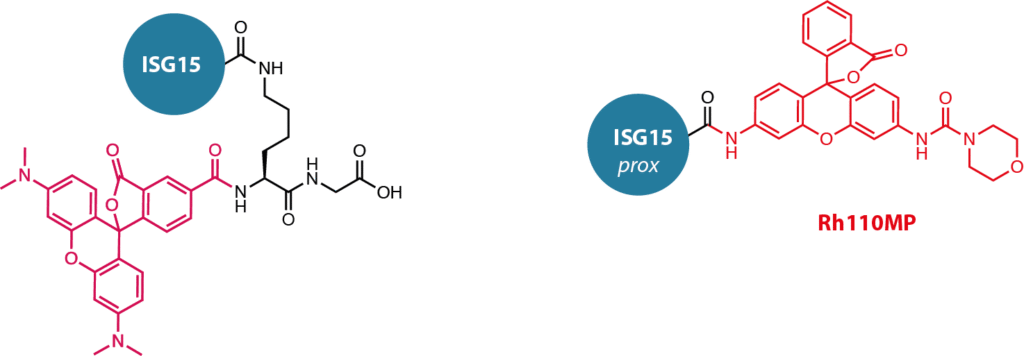
Figure 1.
Activity-based probes. Here we offer UbiQ-262, based on the C-terminal domain of mouse ISG15 and UbiQ-311, based on the C-terminal domain of human ISG15 (Figure 2). The probes contain a C-terminal vinyl pentynyl sulfone (VPS) electrophile, allowing for post-labeling modification of cross-linked complexes by using click chemistry.
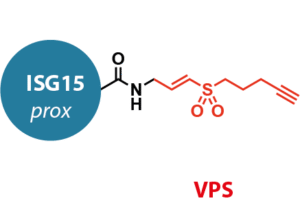
Figure 2.
