news
Regulation of the endosomal SNX27-retromer by OTULIN.
Collaborative research between scientists at the Helmholtz Zentrum, Medical Research Council, University of Oxford, UbiQ and the Walter and Eliza Hall Institute is published in Nature Communications and outlines the identification of the deubiquitinating enzyme OTULIN as a regulator of the levels of nutrient transporters on the cell surface. This insight is important for potential treatments of metabolic diseases.
OTULIN (OTU Deubiquitinase With Linear Linkage Specificity) specifically hydrolyzes Met1-linked ubiquitin chains conjugated by LUBAC (linear ubiquitin chain assembly complex). Using mass spectrometric identification, the OTULIN interactor SNX27 (sorting nexin 27) was identified as an adaptor of the endosomal retromer complex responsible for protein recycling to the cell surface. The C-terminal PDZ-binding motif (PDZbm) in OTULIN associates with the cargo-binding site in the PDZ domain of SNX27. By solving the structure of the OTU domain in complex with the PDZ domain, we demonstrate that a second interface contributes to the selective, high affinity interaction of OTULIN and SNX27. SNX27 does not affect OTULIN catalytic activity, OTULIN-LUBAC binding or Met1-linked ubiquitin chain homeostasis. However, via association, OTULIN antagonizes SNX27-dependent cargo loading, binding of SNX27 to the VPS26A-retromer subunit and endosome-to-plasma membrane trafficking. Overall, our research defines an additional, non-catalytic function of OTULIN in the regulation of SNX27-retromer assembly and recycling to the cell surface.
To identify potential OTULIN interactors by mass spectrometry, UbiQ developed the biotinylated linear di-ubiquitin activity-based probe biotin-Ahx-Ub(1-75)-Dha-Ub (UbiQ-121), which covalently labels with high selectivity active OTULIN in cell extracts.
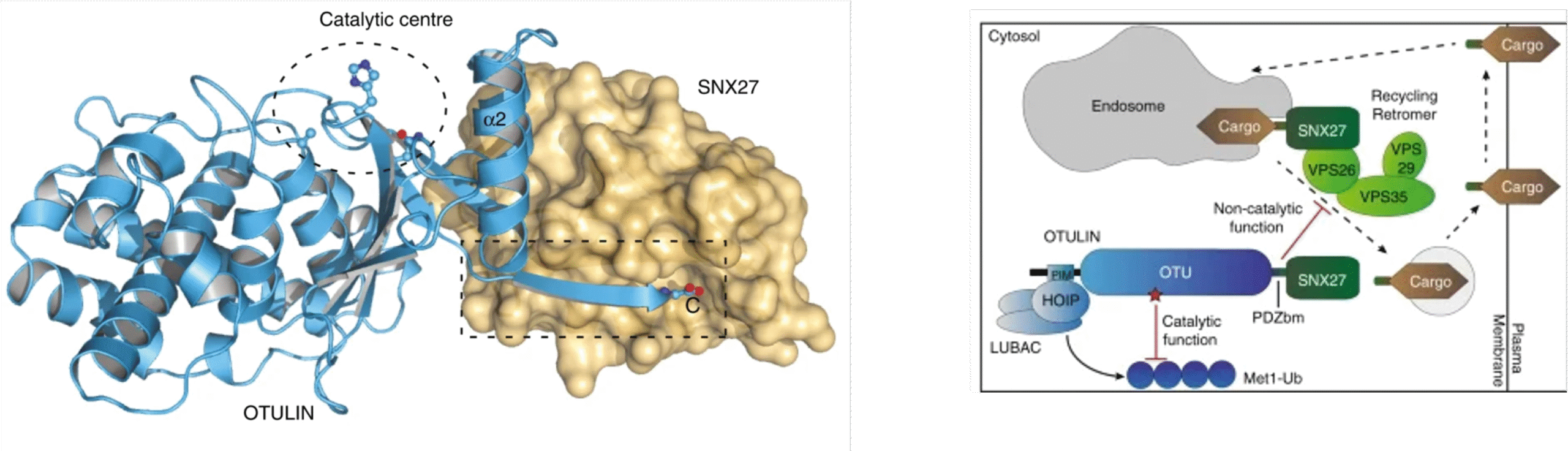
Figure 1. Left: structure of the OTULIN-SNX27 complex revealing the canonical PDZ-PDZbm interface. Structure of OTULIN (blue cartoon) bound to SNX27 (brown surface). The OTULIN catalytic center and also the OTULIN PDZbm are highlighted (dotted lines). Right: schematic model for the dual function of OTULIN. Through LUBAC binding and catalytic activity OTULIN controls Met1-ubiquitin chain homeostasis. By binding to SNX27 OTULIN counteracts cargo recruitment and retromer assembly to antagonize endosome-to-plasma membrane trafficking of internalized cargos.
