news
A cascading activity-based probe sequentially targets E1–E2–E3 enzymes – Triple E technology
UbiQ is proud to be part of a collaborative project reported in Nature Chemical Biology (2016, 12, 523). This paper outlines the development of our cascading activity-based probe for E1-E2-E3 enzymes (UbiQ Triple E technology, Figure 1).
Abstract. Post-translational modifications of proteins with ubiquitin (Ub) and ubiquitin-like modifiers (Ubls), orchestrated by a cascade of specialized E1, E2 and E3 enzymes, control a wide range of cellular processes. To monitor catalysis along these complex reaction pathways, we developed a cascading activity-based probe, Ub-Dha. Similarly to native Ub, upon ATP-dependent activation by the E1, Ub-Dha can travel downstream to the E2 (and subsequently E3) enzymes through sequential trans-thioesterifications. At physiological pH the Dha group is relatively inert. Unlike native Ub, at each step along the cascade, Ub-Dha has the option to react irreversibly with active site cysteine residues of target enzymes, thus enabling their detection. We show that our cascading probe ‘hops’ and ‘traps’ catalytically active Ub-modifying enzymes (but not their substrates) by a mechanism diversifiable to Ubls. Our founder methodology, amenable to structural studies, proteome-wide profiling and monitoring of enzymatic activity in living cells, presents novel and versatile tools to interrogate Ub and Ubl cascades.

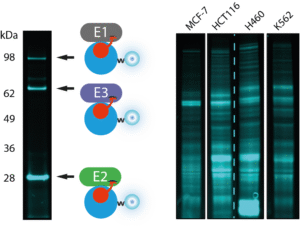
Figure 1. Left: Mode of action Triple E probes. Right: In-gel fluorescence imaging of a mixture of E1 (Uba1), E2 (Ube2L3) and E3 (ITCH) and a panel of tumor cell lines, all incubated with UbiQ-104 (Cy5-Ub-Dha) and ATP.
Our Triple E 
- UbiQ-101 : Ub-Dha
- UbiQ-102 : Biotin-Ahx-Ub-Dha
- UbiQ-103 : His6-Ahx-Ahx-Ub-Dha
- UbiQ-104 : Cy5-Ub-Dha
- UbiQ-131 : 5-carboxyRh110-Ub-Dha
- UbiQ-105 : Nedd8-Dha
- UbiQ-106 : Biotin-Ahx-Nedd8-Dha
- UbiQ-122 : 5-carboxyRh110-Nedd8-Dha
- UbiQ-116 : SUMO1-Dha
- UbiQ-130 : 5-carboxyRh110-SUMO1-Dha
- UbiQ-117 : SUMO2-Dha
- UbiQ-159 : Biotin-Ahx-SUMO2-Dha
- UbiQ-118 : SUMO3-Dha
- UbiQ-124 : His10-SUMO3-Dha
- UbiQ-158: His6-3C-MAP1LC3a-Dha
FAQ.
- Which of the E1-E2-E3 enzymes is targeted by the Triple E probe? The probe targets active site cysteine based E1-E2-E3 enzymes.
- Is the probe transferred to substrates of the E1-E2-E3 enzymes? As far as we know the Triple E Probe is not transferred to substrates.
- How specific is the probe labelling? The ubiquitin(-like) substrate context of the probe in combination with the required activation by the E1, makes Triple E probes highly specific for E1-E2-E3 enzymes. For a complete background of probe reactivity please see Mulder et al.
- Netherlands Cancer Institute, Amsterdam, the Netherlands.
- Medical Research Council Laboratory of Molecular Biology, Cambridge, UK.
- St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
- Leiden University Medical Center, Leiden, the Netherlands.
- University of Konstanz, Konstanz, Germany.
- Howard Hughes Medical Institute, Memphis, Tennessee, USA.
- UbiQ Bio BV, Amsterdam, the Netherlands.
- These authors contributed equally to this work.
