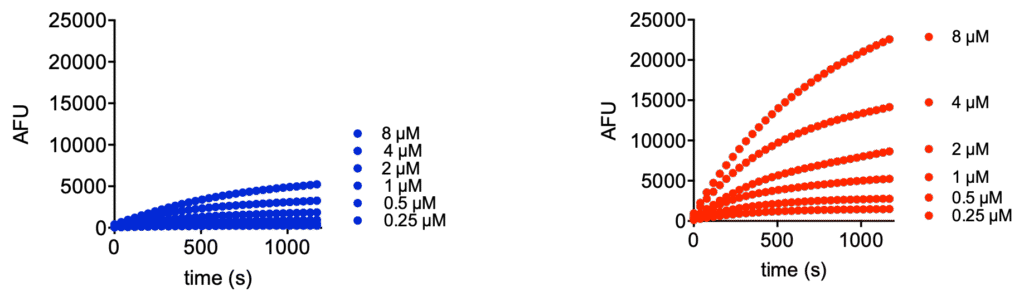news
Introducing Ub-Rh110MP (UbiQ-126) as a new and powerful DUB activity assay reagent
We would like to introduce Ub-Rh110MP (UbiQ-126, Figure 1) as a new quenched ubiquitin rhodamine110 assay reagent for deubiquitinating enzymes (DUBs). UbiQ-126 is based on ubiquitin functionalized with a C-terminal rhodamine110-morpholinecarbonyl group (Rh110MP). Cleavage of the amide bond between G76 of ubiquitin and Rh110MP releases the highly fluorescent Rh110MP, (exc 492 nm, abs 525 nm) which exhibits a much higher fluorescence intensity than the classical Rh110Gly residue used in UbiQ-002. Figures 2 – 4 depict performance data of UbiQ-126.
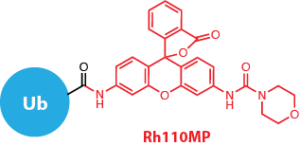
Figure 1.
Overall, UbiQ-126 has:
- the same excellent properties as the classic Ub-Rh110Gly substrate and
- increased fluorescence intensity upon DUB cleavage
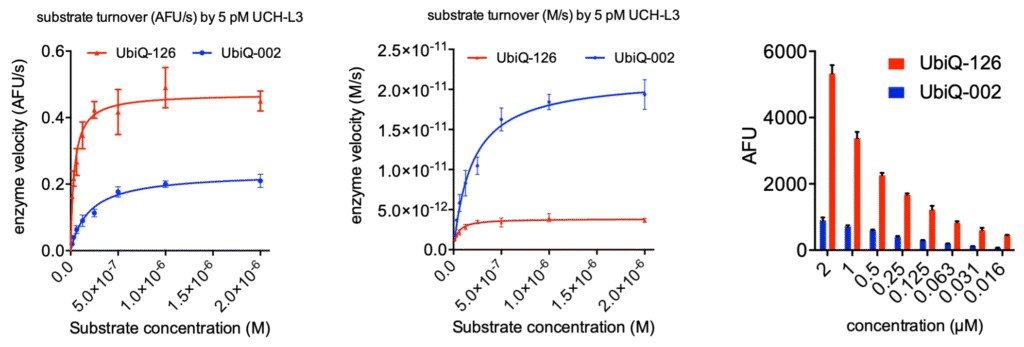
Figure 2. Michaelis-Menten kinetics of UbiQ-126 and UbiQ-002 (Ub-Rh110Gly), turned over by 5 pM UCH-L3. Kinetics were determined in 384 well format (30 uL per well) on a BMG Clariostar plate reader measuring fluorescence intensity at lexc 487 ± 14 nm; lemi 535 ± 30 nm; 40 flashes per well. Left: enzyme velocity represented as AFU/s versus substrate concentration. AFU: arbitrary fluorescence units. Middle: enzyme velocity represented as M/s versus substrate concentration. Right: fluorescence intensities determined at 30 min turnover by 5 pM UCH-L3, error bars are SD (n=3).
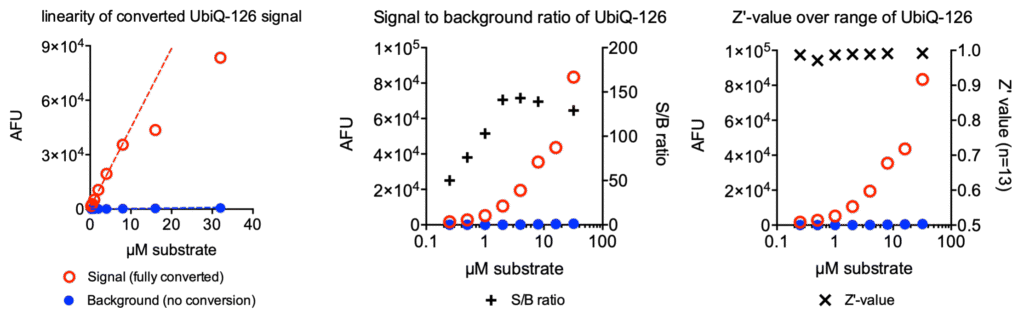
Figure 3. Fluorescence signal versus background of UbiQ-126. Fluorescence intensities were measured of various concentrations of UbiQ-126 (background) and fully converted UbiQ-126 by 1 µM USP7 (signal). Left: the fluorescence signal of processed UbiQ-126 is linear up to 8 µM. Middle: signal-to-background ratios over a concentation range of UbiQ-126. Right: Z’-values over a concentation range of UbiQ-126, determined over 13 replicates. Fluorescence intensities were measured in 384 well format on a BMG Pherastar plate reader at lexc 485 ± 16 nm; lemi 520 ± 10 nm.