news
Diubiquitin-based NMR analysis: Interactions Between Lys6-Linked diUb and the UBA Domain of UBXN1
UbiQ is proud to have contributed to collaborative research carried out by The Netherlands Cancer Institute, Leiden University Medical Centre, Utrecht University, UbiQ and the University of Cologne.
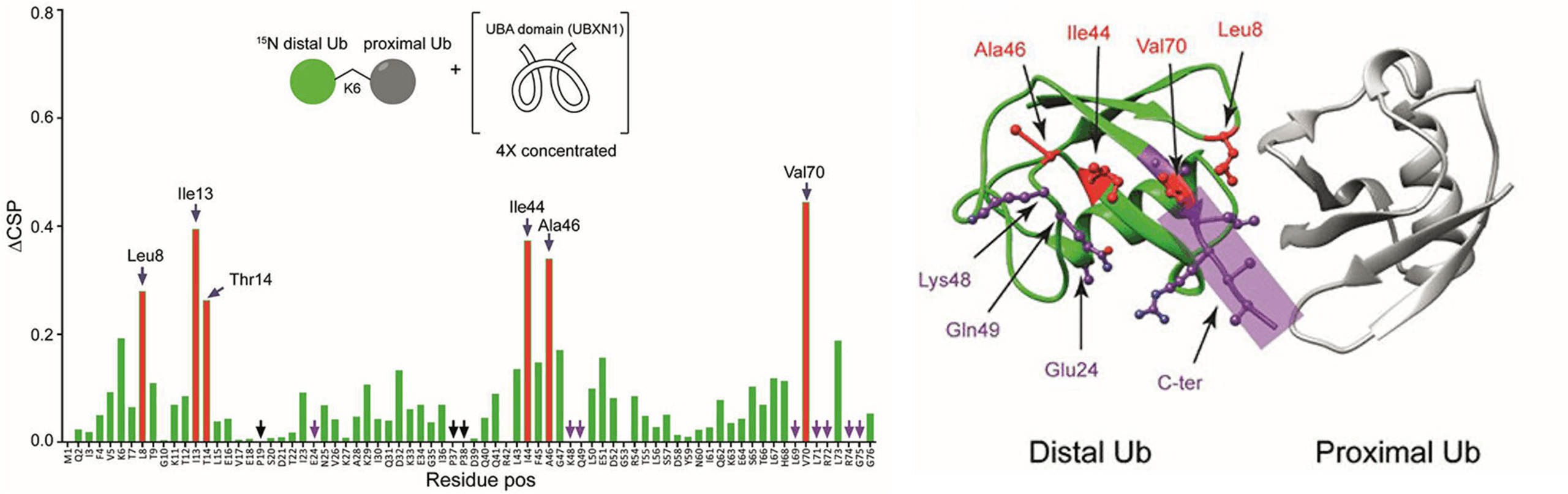
Here Hameed et al. report in Frontiers in Chemistry the synthesis of diubiquitin chains with a fully 15N-labeled distal ubiquitin. Next, advanced NMR spectroscopy was used to confirm that diubiquitin proteins adopt different conformations in solution. This structural flexibility is important in binding with ubiquitin-binding domains (UBD) thereby inducing unique responses. One of the well-known but poorly understood UBD-Ub interactions is the recognition of K6-linked ubiquitin chains by the ubiquitin-associated (UBA) domain of UBXN1 in the BRCA-mediated DNA repair pathway (Figure 1). Using 15N-labeled K6-linked diubiquitin, the C-terminally extended UBA domain of UBXN1 was shown to confer specificity to K6-linked diubiquitin while the non-extended version of the domain does not show any linkage preference. Thus, the two distinct conformations of K6-linked diubiquitin that exist in solution converge into a single conformation upon binding to the UBA domain of the UBXN1 protein. It is likely that more of such extended UBA domains exist in nature and can contribute to linkage-specificity in ubiquitin signaling.
Figure 1. Left: Unlabeled UBA(ext1-52) domain of UBXN1 was added in different concentrations to 15N-K6 diUb and the CSPs were monitored. At a ratio of 4:1 (UBA(ext1–52) domain:K6 diUb), residues Leu8, Ile44, Ala46, and Val70 (red bars, labeled) shifted more than the rest. Other residues like Tyr 59 remain unchanged. Right: X-ray crystal structure of a K6 diUb (PDB: 2XEW) showing the residues that were perturbed according to CSP. Residues that shifted more are colored in red. Residues whose signal disappeared upon addition of UBA(ext1-52) peptide are represented in purple. Several perturbed residues are found to be positioned on the surface away from proximal Ub.