news
Catalytic and regulatory mechanisms of the dual-specificity ubiquitin/FAT10 E1 enzyme Uba6
UbiQ is proud to have been part of a major collaborative research project focused on studying the dual-specificity ubiquitin/FAT10 E1 enzyme Uba6 and whose results have been published in Nature Communications.
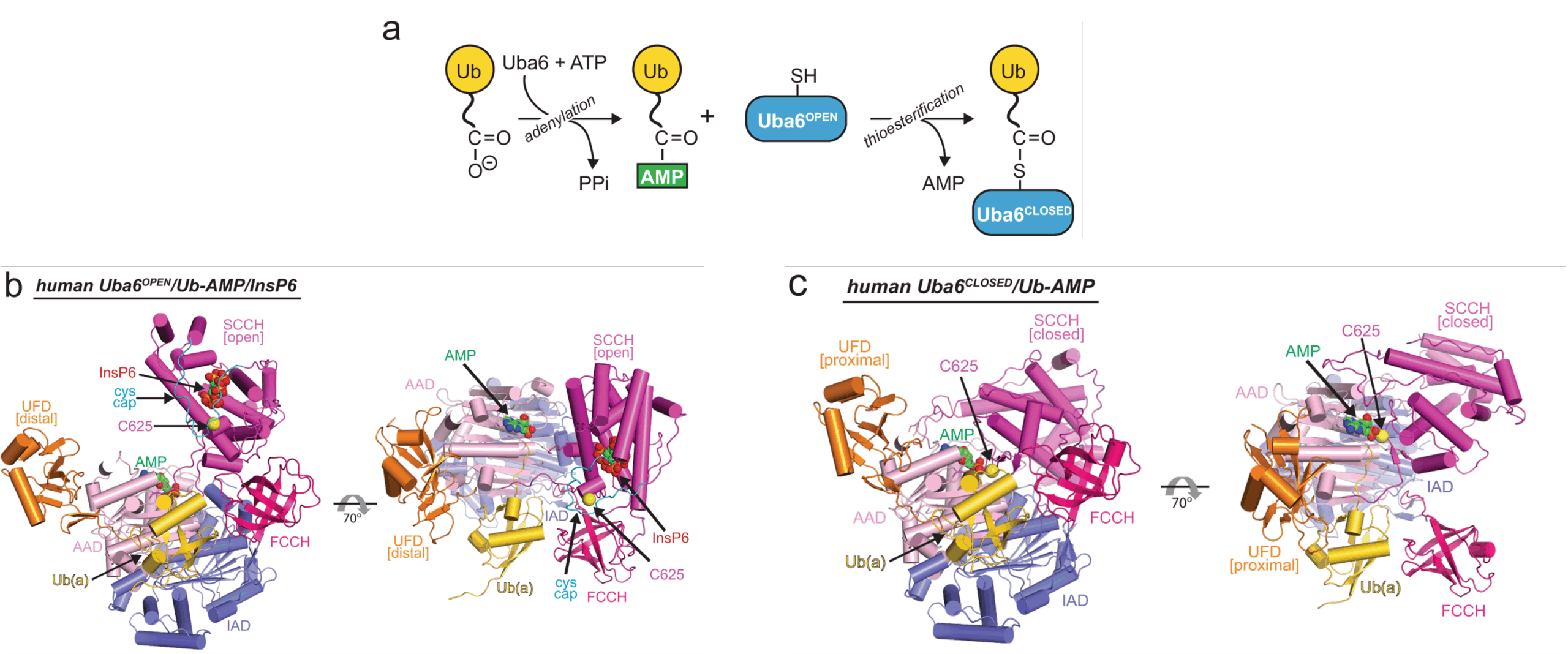
Uba6 is an E1 enzyme that initiates signal transduction by activating ubiquitin and the ubiquitin-like protein FAT10 in a two-step process involving sequential catalysis of adenylation and thioester bond formation. By determining the crystal structure of a human Uba6-ubiquitin complex, two distinct architectures were observed: one in which Uba6 adopts an open conformation (with the active site configured for catalysis of adenylation), and a second drastically different closed conformation in which the adenylation active site is disassembled and reconfigured for catalysis of thioester bond formation. Surprisingly, one molecule of the important cellular metabolite inositol hexakisphosphate (InsP6) was found to bind a previously unidentified allosteric site on Uba6. Our data indicate that InsP6 binding not only enhances Uba6 stability but also inhibits its activity by altering interconversion of the open and closed conformation. Thus, in addition to revealing the molecular mechanisms of catalysis by Uba6 and allosteric regulation of its activities, our data* provide a framework for developing Uba6-specific inhibitors and raise the possibility of allosteric regulation of other E1s by naturally occurring cellular metabolites.