news
Quantification of DUB activity with fluorescent probes
Immunoblotting procedures using ubiquitin probes with an affinity tag are widely used to monitor DUB inhibition in DUB inhibitor potency and specificity profiling studies. Here we would like to introduce fluorescent DUB activity-based probes with many advantages:
- time saving (direct read-out)
- reliable, reproducible quantification of DUB activity
- sensitive and high resolution
- a large linear window for quantifications
- no background labelling from antibody
Figure 1A and 1B depict a comparison between using western blotting and in-gel fluorescence scanning for DUB activity-based profiling in cell lysate – the differences are obvious. One of the main advantages of fluorescent probes is that they enable quantitative assessment of genetic or chemical inhibition of DUB activity. Figure 1C shows DUB activity profiling of the small-molecule DUB inhibitor b-AP15 in EL4 (mouse lymphoma) cell lysate, using our fluorescent probe TAMRA-Ub-VME (UbiQ-050).
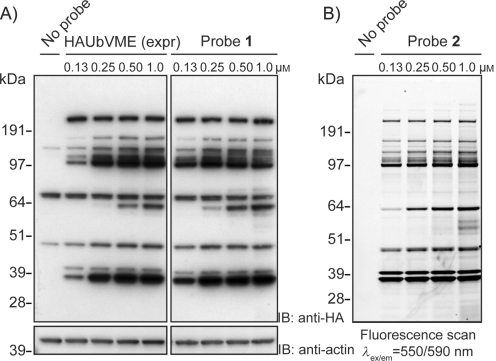
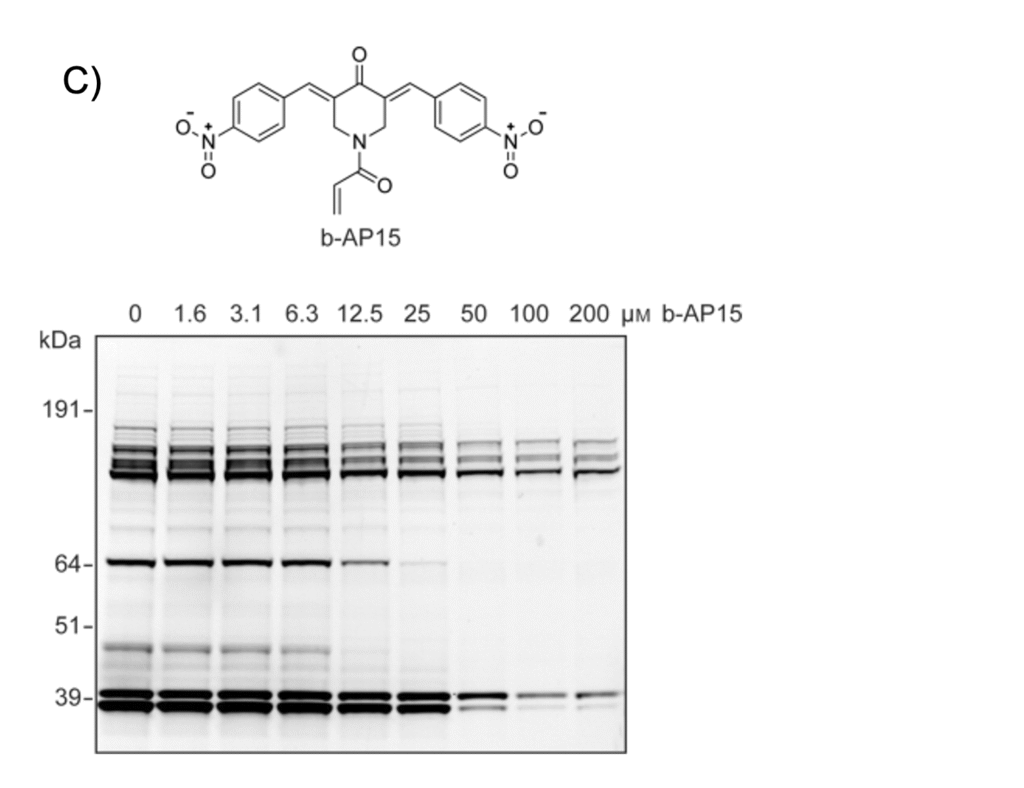
Figure 1. A) EL4 cell lysate incubated with HA-Ub-VME (probe 1, obtained by the conventional intein method) – proteins were separated by SDS-PAGE and analyzed by immunoblotting. Actin, loading control. B) EL4 lysate incubated with fluorescent probe 2 (TAMRA-Ub-VME, UbiQ-050). Proteins were separated by SDS-PAGE and analyzed by in-gel fluorescence scanning. C) EL4 lysate was incubated with the indicated concentrations of small-molecule DUB inhibitor b-AP15 (top). Subsequently, lysate was labeled with probe 2 (TAMRA-Ub-VME, UbiQ-050). Proteins were separated by SDS-PAGE and the residual DUB activity was visualized by in-gel fluorescence scanning (bottom). From: de Jong et al. ChemBiochem 2012, 13, 2251.

