news
USP1/UAF1 targets polyubiquitinated PCNA with an exo-cleavage mechanism
UbiQ is proud to have contributed to this paper in Nature Communications, describing our scientific collaboration on USP1 and PCNA with the Netherlands Cancer Institute. If you are interested in DNA damage response, DUBs, advanced tool development, and cryo-EM (Figure 1), please check out the paper and have a look at the LinkedIn post of our collaborator and NKI scientist Niels Keijzer.
DNA damage tolerance is an important pathway by which cells bypass DNA lesions during replication. It is orchestrated by ubiquitination of PCNA, where monoubiquitinated PCNA initiates recruitment of translesion synthesis polymerases but also serves as a substrate for further ubiquitination, forming K63-polyubiquitinated PCNA that leads to homologous recombination-mediated bypass mechanisms. Recent work on USP1/UAF1 inhibition revealed that formation of K48-linked ubiquitin chains also occurs on PCNA, resulting in its proteasomal degradation. USP1/UAF1 is established as the deubiquitinating enzyme (DUB) for PCNA-ubiquitin, but little is known about removal of ubiquitin chains on PCNA. Here we show that USP1/UAF1 cleaves both K48 and K63-linked ubiquitin chains on PCNA efficiently, using an exo-cleavage mechanism. Kinetic analysis reveals that USP1/UAF1 prefers cleaving the ubiquitin-ubiquitin isopeptide bond over cleavage of the ubiquitin-PCNA bond and therefore treats poly- and monoubiquitinated PCNA as different substrates. A cryo-EM structure of USP1/UAF1 with K63-diubiquitin probe UbiQ-087 (Figure 1) and structure-based mutagenesis suggests that this mechanistic preference is maintained in evolution. Overall, the unusual mechanism we present, can cause temporal enrichment of monoubiquitinated PCNA during polyubiquitination of PCNA. It will be interesting to see how this affects the balance in the DNA damage tolerance pathway.
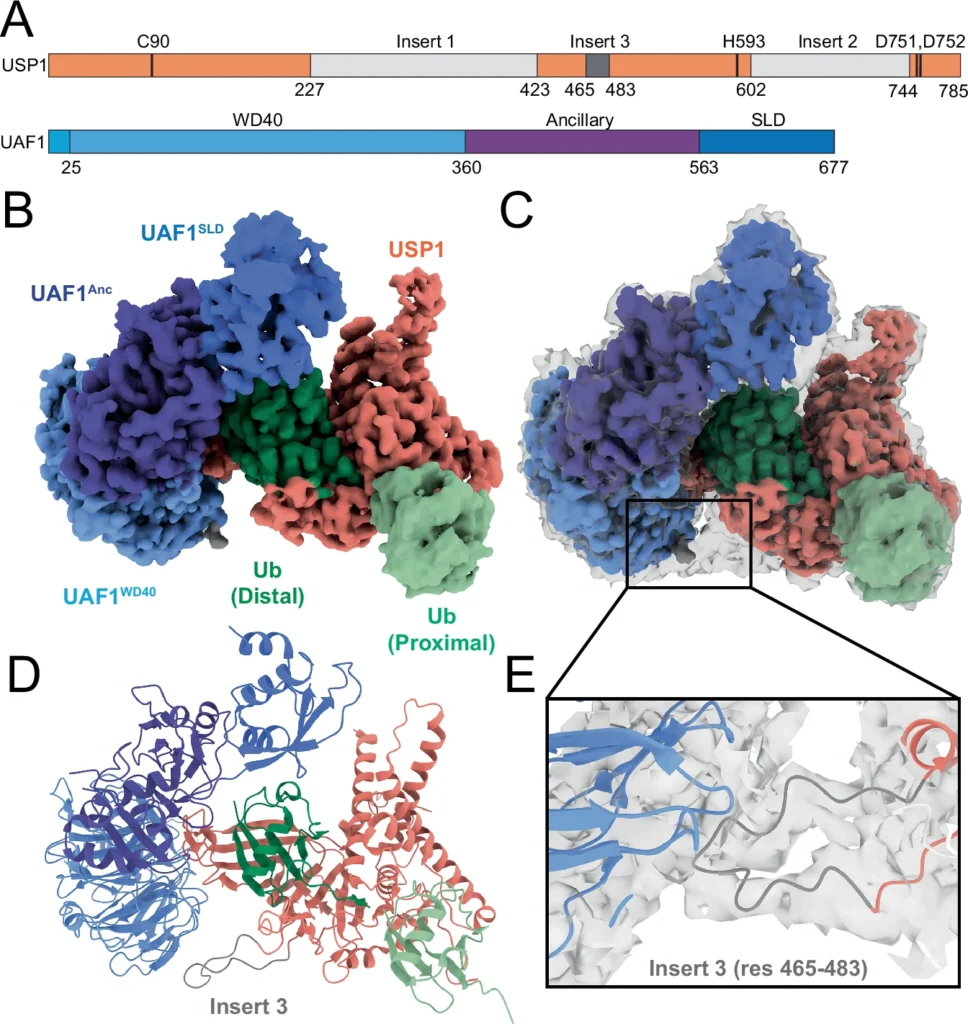
Figure 1. Cryo-EM structure of USP1/UAF1 bound to activity-based probe UbiQ-087.
A: Domain architecture of USP1 and UAF1. Density was resolved for USP1’s insert 3 (dark gray), but not for insert 1 and insert 2 (light gray). B: Final map of 3.0 Å final structure. DeepEMhancer sharpened map is colored as in A and shown at threshold 0.005. C: DeepEMhancer sharpened map from (B), placed in the non-sharpened final density map (gray transparent map) shown at threshold 0.015 to accentuate density for flexible regions. D: Atomic reconstruction of the structure of USP1/UAF1 bound to K63-linked probe. E: Zoom showing the backbone of insert 3 of USP1 in the non-sharpened map shows how it anchors at the WD40 domain of UAF1.
