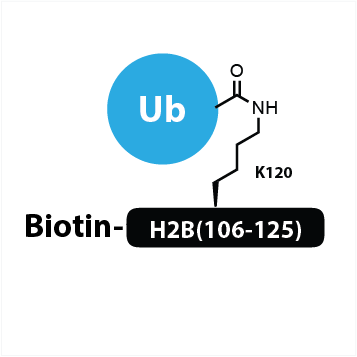
Biotin-Ahx-H2B(106-125) K120Ub
an H2B(106-125) peptide which is modified at K120 via a native isopeptide bond with ubiquitin and modified on the N-terminus with biotin
Additional information
| Weight | 0.05 kg |
|---|---|
| aliquot size | |
| Applications | Crystallization, Pull down, Western Blot, Phenotypic protein profiling, DUB activity profiling, Target inhibitor profiling |
| target | |
| source | human sequence, synthetic |
| shipping | |
| purity | |
| molecular weight | |
| storage | |
| sample preparation | For detailed sample preparation see product sheet. |
| regulatory statement |
€300.00
- Description
- Additional information
- references
Description
UbiQ-150 is an H2B(106-125) peptide which is modified at K120 via a native isopeptide bond with ubiquitin (Ub) and modified on the N-terminus with biotin. An aminohexanoic acid (Ahx) linker is used to create extra space between the biotin and H2A peptide for efficient access of biotin binding entities. It can be used as a substrate for ubiquitin proteases, to investigate mechanism of binding and recognition by proteins that contain ubiquitin-associated domains or ubiquitin-interacting motifs (UIMs) and as antigen for immunizations.
Additional information
| Weight | 0.05 kg |
|---|---|
| aliquot size | |
| Applications | Crystallization, Pull down, Western Blot, Phenotypic protein profiling, DUB activity profiling, Target inhibitor profiling |
| target | |
| source | human sequence, synthetic |
| shipping | |
| purity | |
| molecular weight | |
| storage | |
| sample preparation | For detailed sample preparation see product sheet. |
| regulatory statement |
F. El Oualid et al.
El Oualid, F., et al. Chemical Synthesis of Ubiquitin, Ubiquitin-Based Probes, and Diubiquitin. Angewandte Chemie Int. Ed. 49, 10149-10153 (2010).
http://www.ncbi.nlm.nih.gov/pubmed/21117055
Faesen et al.
Faesen, A.C., et al. The Differential Modulation of USP Activity by Internal Regulatory Domains, Interactors and Eight Ubiquitin Chain Types. Chem. Biol. 18, 1550-1561 (2011).
http://www.ncbi.nlm.nih.gov/pubmed/22195557
I. Dikic et al.
Dikic, I., et al. Ubiquitin-binding domains – from structures to functions. Nat. Rev. Mol. Cell. Biol. 10, 659-671 (2010).
http://www.ncbi.nlm.nih.gov/pubmed/19773779
J. D. F. Licchesi et al.
Licchesi, J.D., et al. An ankyrin-repeat ubiquitin-binding domain determines TRABID’s specificity for atypical ubiquitin chains. Nat. Struct. Mol. Biol. 19, 62-71 (2012).
http://www.ncbi.nlm.nih.gov/pubmed/22157957
