news
Metabolic control of BRISC–SHMT2 assembly regulates immune signalling
UbiQ is proud to be part of an international collaboration led by Dr Elton Zeqiraj (University of Leeds, UK) and Prof Roger Greenberg (University of Pennsylvania, US) that reports in Nature (2019, 570, 194) the cryo-electron microscopy structure of the human BRISC–SHMT2 complex (Figure 1). This complex is involved in cell metabolism and immune response. The structure showed that (inactive dimeric) SHMT2 inhibits the BRCC36 deubiquitylase activity of BRISC by steric hindrance of the BRCC36 active site. Since pyridoxal-5′-phosphate (PLP, the active form of vitamin B6) stabilizes SHMT2 in an active tetrameric state, the study identified an important mechanism in which a metabolite (PLP) regulates immune signalling by interfering with ubiquitin signalling (i.e. BRISC activity). This insight is important for potential treatments of metabolic and auto-immune diseases.
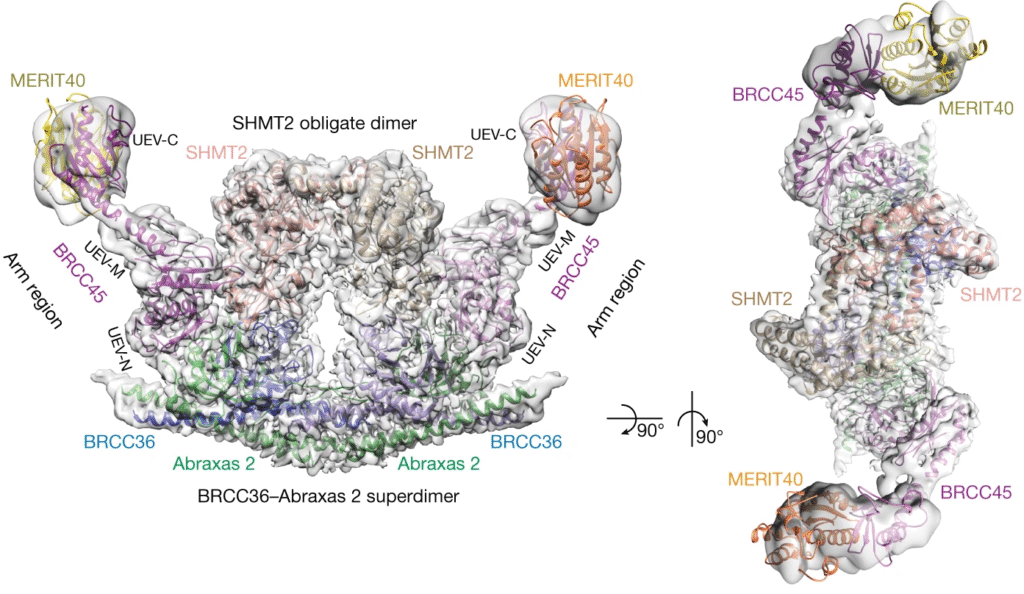
Figure 1. cryo-EM structure of the BRISC–SHMT2 complex.

- Astbury Centre for Structural Molecular Biology, School of Molecular and Cellular Biology, Faculty of Biological Sciences, University of Leeds, Leeds, UK.
- Department of Cancer Biology, Basser Center for BRCA, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
- Leeds Institute of Rheumatic and Musculoskeletal Medicine and NIHR Biomedical Research Centre, University of Leeds, Leeds, UK.
- Department of Biochemistry, Institute of Integrative Biology, University of Liverpool, Liverpool, UK.
- The Wistar Cancer Center for Molecular Screening, The Wistar Institute, Philadelphia, PA, USA.
- UbiQ Bio BV, Amsterdam, The Netherlands.
- Warsaw University of Life Sciences, Warsaw, Poland.
- Department of Translational Medicine, Clinical Sciences, Lund University, Lund, Sweden.
- These authors contributed equally: Miriam Walden, Lei Tian.
