news
Kinetic analysis of multistep USP7 mechanism shows critical role for target protein in activity
UbiQ is proud to be part of a collaborative project reported in Nature Communications (2019, 10, article 231) which describes a detailed mechanistic study of the deubiquitinating enzyme USP7. USP7 is a highly abundant deubiquitinating enzyme (DUB), involved in cellular processes including DNA damage response and apoptosis. Because USP7 plays important roles in DNA damage response and apoptosis, understanding how it achieves specificity for its many cellular targets is important for future clinical development of USP7 inhibitors. USP7 has an unusual catalytic mechanism, where the low intrinsic activity of the catalytic domain (CD) increases when the C-terminal Ubl domains (Ubl45) fold onto the CD, allowing binding of the activating C-terminal tail near the catalytic site. Here we delineate how the target protein promotes the activation of USP7. Using NMR analysis and biochemistry we describe the order of activation steps, showing that ubiquitin binding is an instrumental step in USP7 activation. Using chemically synthesised p53-peptides we also demonstrate how the correct ubiquitinated substrate increases catalytic activity. We then used transient reaction kinetic modelling to define how the USP7 multistep mechanism is driven by target recognition. Our data show how this pleiotropic DUB can gain specificity for its cellular targets.
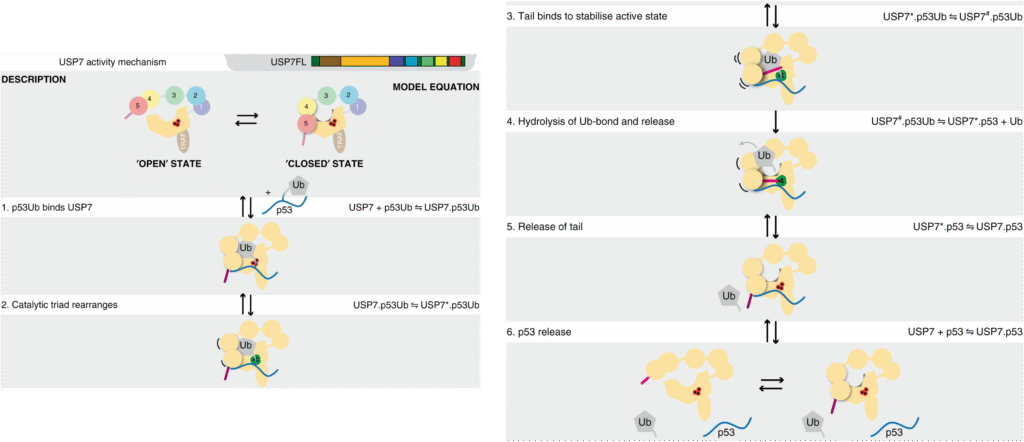
Figure 1. Kinetic model for USP7 mechanism on p53Ub The kinetic model (equations) and their interpretation are depicted schematically. The weak affinity between Ubl45 and CD suggests that free USP7 is in equilibrium between an ‘open’ and ‘closed’ state. The p53Ub substrate is bound by TRAF and CD, as well as an additional binding site that depends on the Ubl domains (1). Ubiquitin binding induces a rearrangement of the catalytic triad33 (2), which dramatically increases the affinity the activating C-terminal tail, but diminishes the contact between CD and Ubl45. Binding of the tail peptide (3) stabilises the active state. This promotes the hydrolysis of the isopeptide bond, allowing Ub release (4). This in turn diminishes affinity for the C-terminal tail, causing its release (5) and the subsequent release of the p53 peptide (6) to return USP7 to the ground state.

- Division of Biochemistry and Oncode Institute, Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, the Netherlands.
- Division of Cell Biology II, Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, the Netherlands.
- UbiQ Bio BV, Science Park 408, 1098 XH Amsterdam, the Netherlands.
- Macromolecular Biochemistry, Leiden Institute of Chemistry, Leiden University, Leiden, the Netherlands.
- Present address: Oncode Institute and Department of Cell and Chemical Biology, Leiden University Medical Center, Leiden, the Netherlands.
- Present address: Tumor Immunology department, Radboud Institute for Molecular Sciences, Nijmegen, the Netherlands.
- Present address: Bijvoet center, Utrecht University, Utrecht, the Netherlands.
