news
Anti–COVID-19 Drug Design by targeting a molecular scissor of Ubiquitin and ISG15
UbiQ is proud to have been part of a major collaboration reported recently by Rut et al. in Science Advances (2020, 6, eabd4596) describing the activity profiling and crystal structures of inhibitor bound SARS-CoV-2 PLpro.
The viral papain-like cysteine protease (PLpro, NSP3) is essential for SARS-CoV-2 replication and represents a promising target for the development of antiviral drugs. Here, we used a combinatorial substrate library and performed comprehensive activity profiling of SARS-CoV-2 PLpro. On the scaffold of the best hits from positional scanning, fluorogenic substrates and irreversible inhibitors were designed with a high degree of selectivity for SARS PLpro. Crystal structures of two of these inhibitors in complex with SARS-CoV-2 PLpro revealed their inhibitory mechanisms, providing a molecular basis for the observed substrate specificity profiles. Last, we demonstrate that SARS-CoV-2 PLpro harbors deISGylating activity similar to SARSCoV-1 PLpro but its ability to hydrolyze K48-linked ubiquitin chains is diminished, which our sequence and structure analysis provides a basis for. Overall, our work reveals the molecular rules governing PLpro substrate specificity and provides a framework for development of inhibitors with potential therapeutic value or drug repurposing.
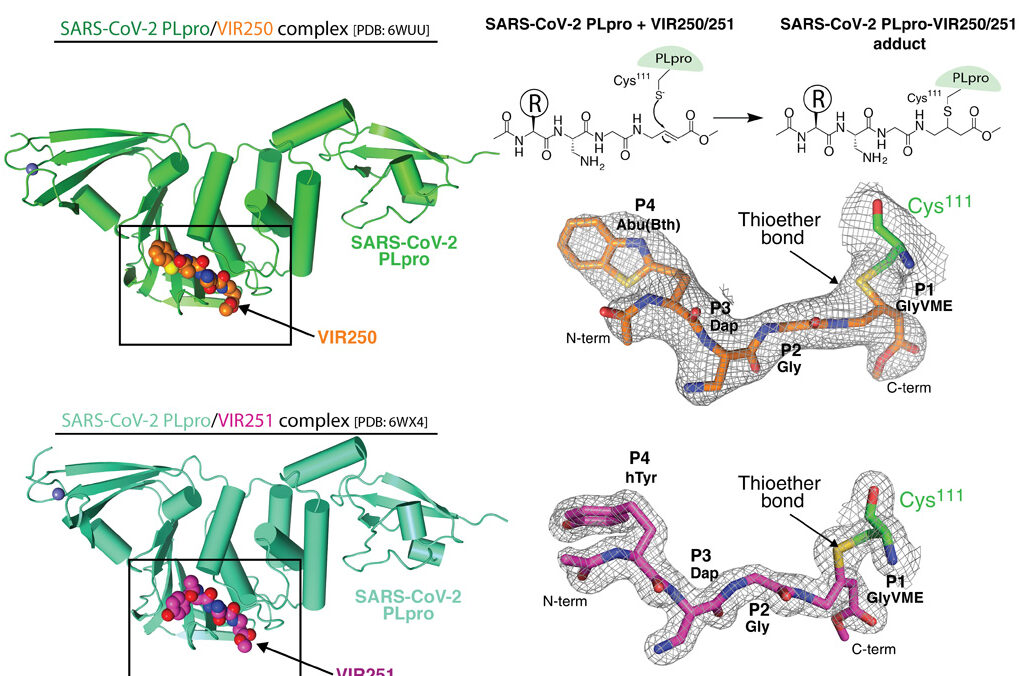
Figure 1. Nonnatural amino acid-containing inhibitors VIR250 and VIR251 and their crystal structures in complex with SARS-CoV-2 PLpro.

- Wroclaw University of Science and Technology, Poland.
- Medical University of South Carolina, Charleston, USA.
- University of Texas Health Science Center at San Antonio, San Antonio, USA.
- New York University School of Medicine, New York, USA.
- Sanford Burnham Prebys Medical Discovery Institute, La Jolla, USA.
- UbiQ Bio B.V., Amsterdam, The Netherlands.
- Independent Consultant.
(*) These authors contributed equally to this work. (†) Corresponding author. (‡) Present address: Arvinas Inc., New Haven, USA.
