news
On terminal alkynes that can react with active-site cysteine nucleophiles in proteases..
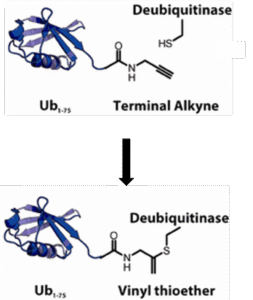
Active-site directed probes are powerful in studies of enzymatic function. In the Journal of the American Chemical Society Ekkebus et al. report activity-based probes based on a new warhead so far considered unreactive: propargyl amide (PA). By replacing the C-terminal carboxylate of ubiquitin (Ub) with an alkyne functionality, a selective reaction with the active-site cysteine residue of de-ubiquitinating enzymes was observed. The resulting product was shown to be a quaternary vinyl thioether, as determined by X-ray crystallography. Proteomic analysis of proteins bound to an immobilized probe confirmed the selectivity toward deubiquitinating enzymes (DUBs).
Figure 1 – propargyl amide as a new warhead for DUB activity-based probes (Ekkebus et al. J Am Chem Soc 2013, 135, 2867).

- UbiQ-057 : Ub-PA
- UbiQ-058 : TAMRA-Ub-PA
- UbiQ-072 : Cy5-Ub-PA
- UbiQ-076 : Biotin-Ahx-Ub-PA
- UbiQ-077: Biotin-ANP-Ub-PA
- UbiQ-078 : HA-Ahx-Ahx-Ub-PA
- UbiQ-168 : Biotin-Ahx-SUMO2-PA