DUB activity-based probe explorer panel
a panel of 10 activity-based probes for deubiquitinating enzymes (DUBs) (10 μg each)
Additional information
| Weight | 0.01 kg |
|---|---|
| Applications | Crystallization, Pull down, Purification, Western Blot, MS, NMR, Phenotypic protein profiling |
| target | |
| source | |
| shipping | |
| storage | upon arrival, powder at −20°C; solution at −80°C. Avoid multiple freeze/thaw cycles. |
| sample preparation | For detailed sample preparation see product sheet. |
| regulatory statement | |
| aliquot size |
€600.00
- Description
- Additional information
- references
Description
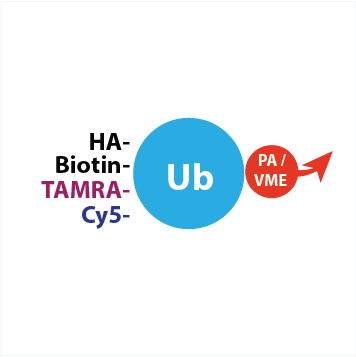
UbiQ-L02 is a panel of the following 10 activity-based probes for deubiquitinating enzymes (DUBs) (10 μg each).
| VME | PA | |||
| tag | code | name | code | name |
| – | UbiQ-005 | Ub-VME | UbiQ-057 | Ub-PA |
| HA | UbiQ-035 | HA-Ahx-Ahx-Ub-VME | UbiQ-078 | HA-Ahx-Ahx-Ub-PA |
| Biotin | UbiQ-054 | Biotin-Ahx-Ub-VME | UbiQ-076 | Biotin-Ahx-Ub-PA |
| TAMRA | UbiQ-050 | TAMRA-Ub-VME | UbiQ-058 | TAMRA-Ub-PA |
| Cy5 | UbiQ-071 | Cy5-Ub-VME | UbiQ-072 | Cy5-Ub-PA |
All probes are prepared by total chemical synthesis. These DUB ABPs are potent, irreversible and specific inhibitors of DUBs which can be used for:
- inhibiting hydrolysis of poly-Ub chains on substrate proteins and thus enhancement of poly-Ub chain accumulation
- structural biology studies of DUB-Ub complexes
- DUB activity profiling experiments
- determine DUB inhibitor specificity
Additional information
| Weight | 0.01 kg |
|---|---|
| Applications | Crystallization, Pull down, Purification, Western Blot, MS, NMR, Phenotypic protein profiling |
| target | |
| source | |
| shipping | |
| storage | upon arrival, powder at −20°C; solution at −80°C. Avoid multiple freeze/thaw cycles. |
| sample preparation | For detailed sample preparation see product sheet. |
| regulatory statement | |
| aliquot size |
Misaghi, S., et al. Structure of the Ubiquitin Hydrolase UCH-L3 Complexed with a Suicide Substrate. J. Biol. Chem. 280, 1512-1520 (2005).
http://www.ncbi.nlm.nih.gov/pubmed/15531586
de Jong, A., et al. Ubiquitin-based probes prepared by total synthesis to profile the activity of deubiquitinating enzymes. ChemBiochem 13, 2251-2258 (2012).
http://www.ncbi.nlm.nih.gov/pubmed/23011887
Altun, M., et al. Activity-based chemical proteomics accelerates inhibitor development for deubiquitylating enzymes. Chem. Biol. 18, 1401-1412 (2011).
http://www.ncbi.nlm.nih.gov/pubmed/22118674
